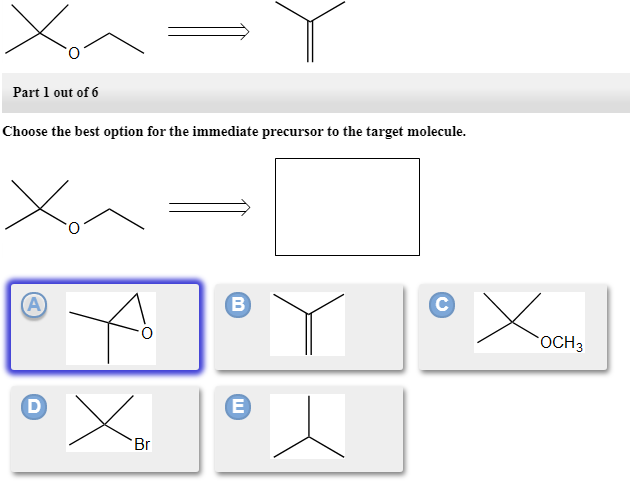
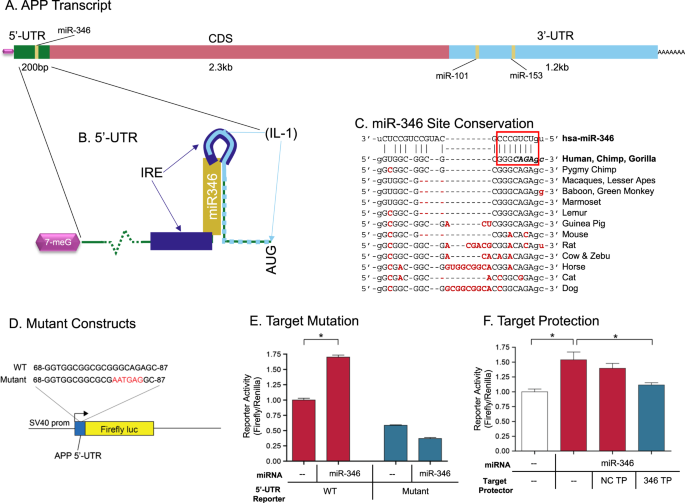
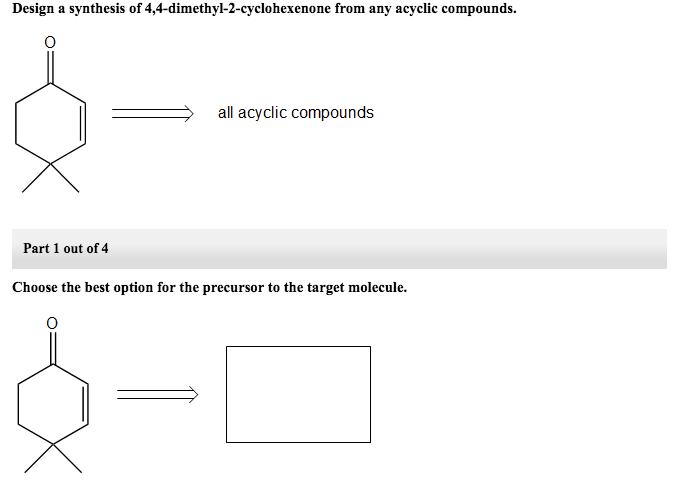
Choose The Best Option For The Immediate Precursor To The Target Molecule.
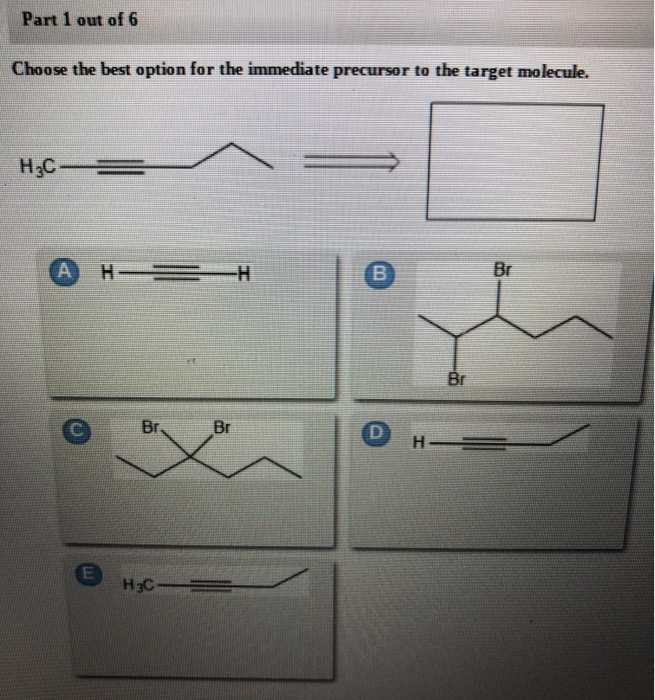
Choose the best option for the immediate precursor to the target molecule.. Synergistic and Competitive Groups Practice Problems. Design a synthesis of 1-butyne from ethyne and ethanol. 2 on a question.
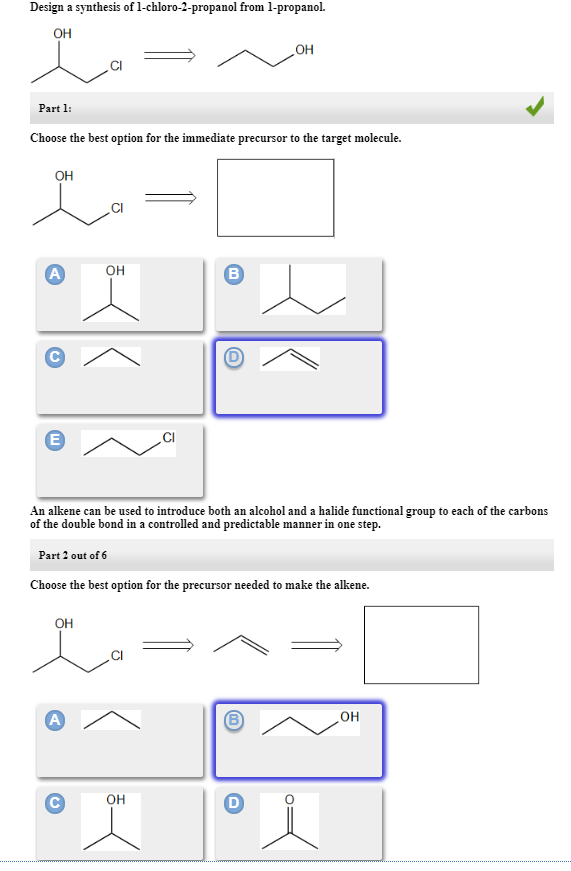
Our videos prepare you to succeed in your college classes. P2 p2d3 p2d2 p2d1 p2c p2d4. An alkene can be used to introduce both an alcohol and a halide functional group to each of the carbons of the double bond in a controlled and predictable manner in one step.
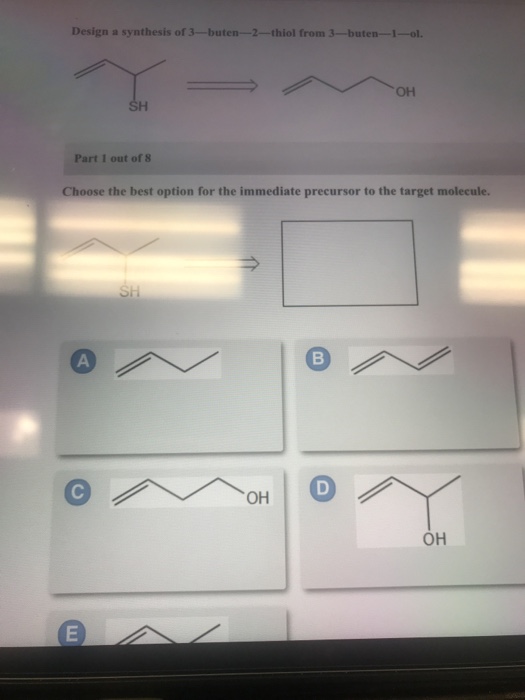
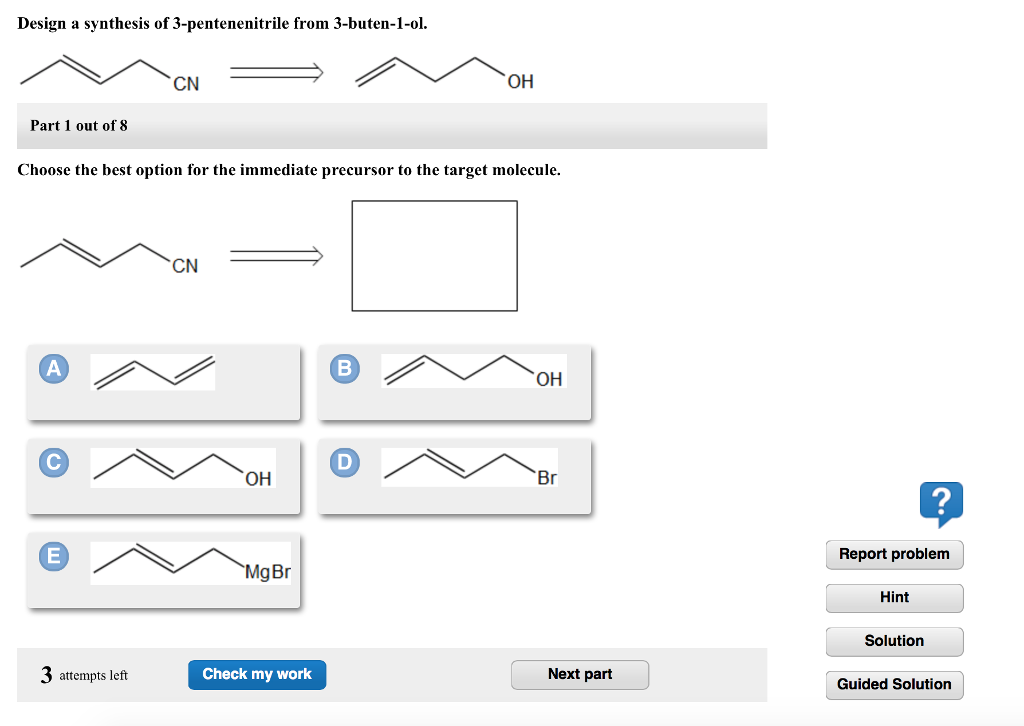
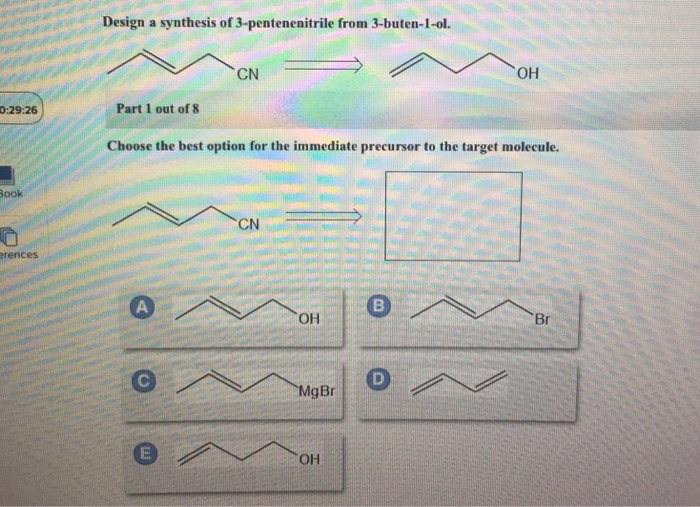
Part 1 out of 6 choose the best option for the immediate precursor to the target molecule. N-butyl bromide 1-butene 2-bromobutane 2-butene. H- HH HO ethyne ethanol Part 1 out of 8 Choose the best option for the immediate nucleophile precursor to the target molecule.
Choose the best option for the immediate precursor to the target molecule. H- Nucleophile Electrophile A B H Na 0 D Na -CH3 Η ΕΘΝa Choose the most appropriate precursor. Choose the best option for the immediate precursor to S-3-iodohexane.
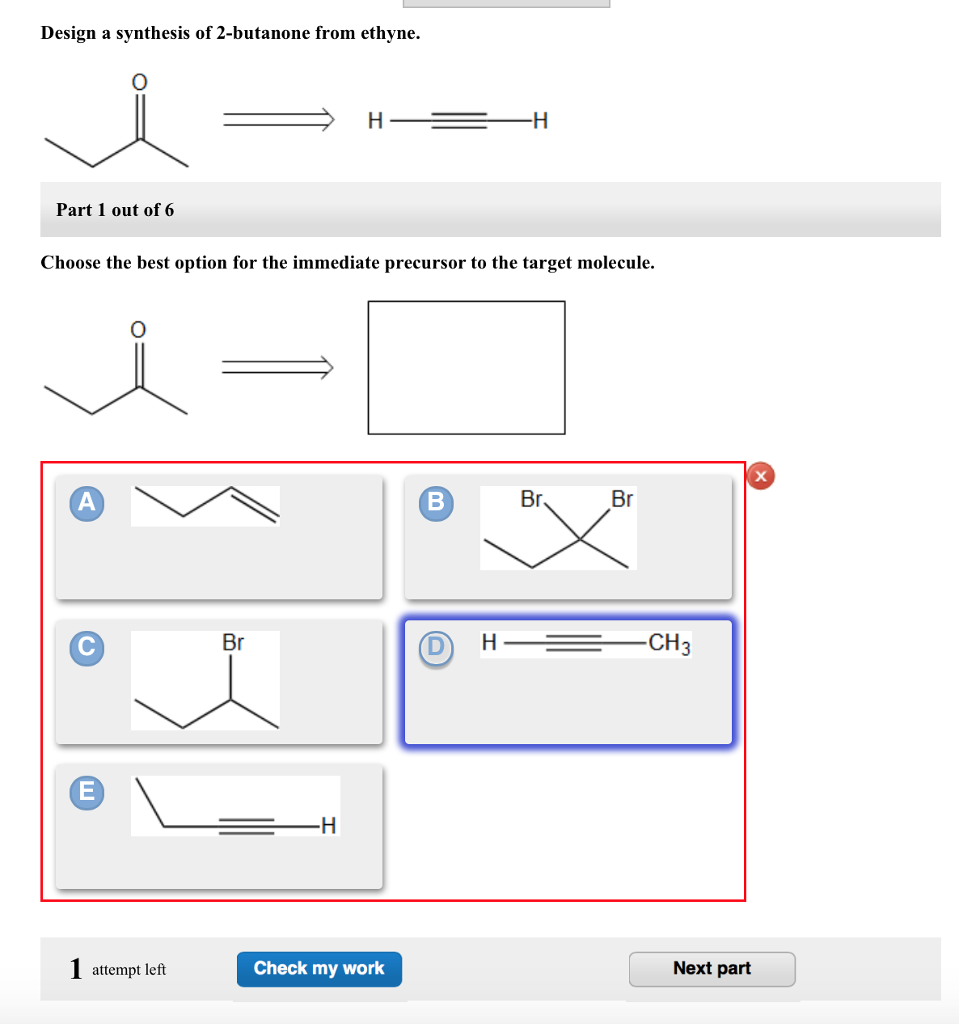
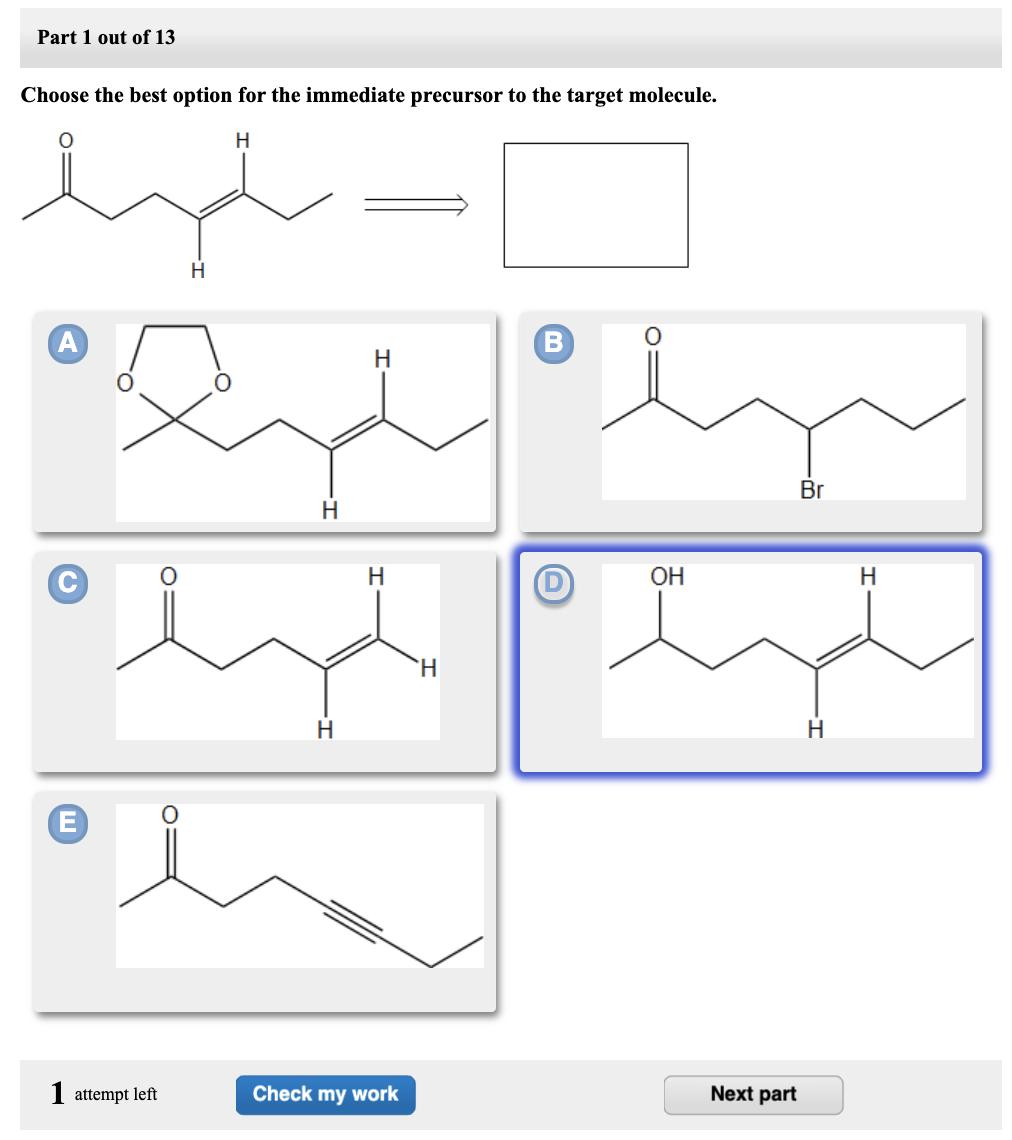
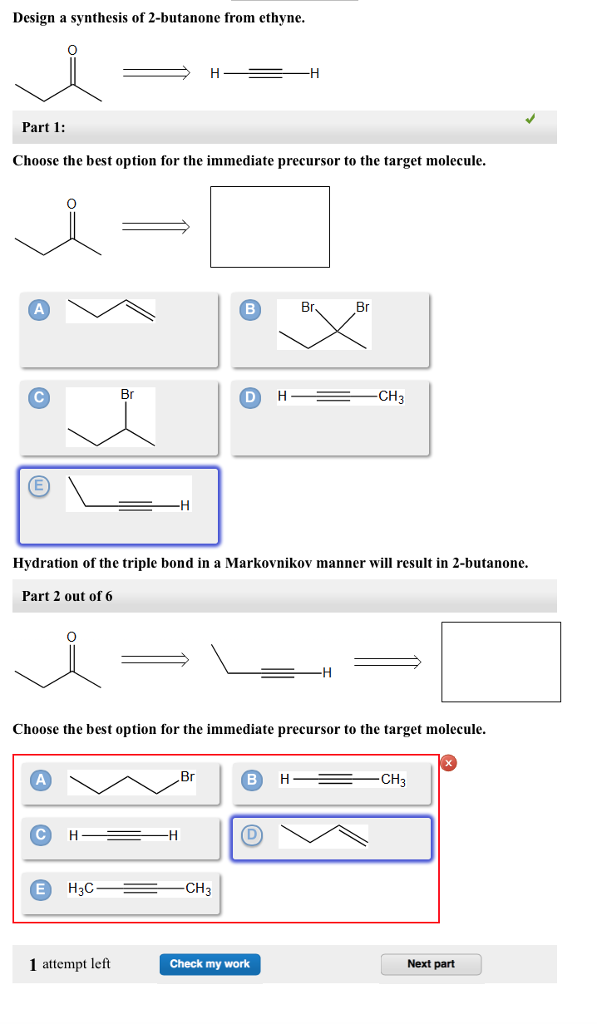
Part 1 Choose the best option for the immediate precursor to the target molecule. Choose the best option for the immediate precursor to the target molecule. Hydration of the triple bond in a Markovnikov manner will result in 2-butanone.
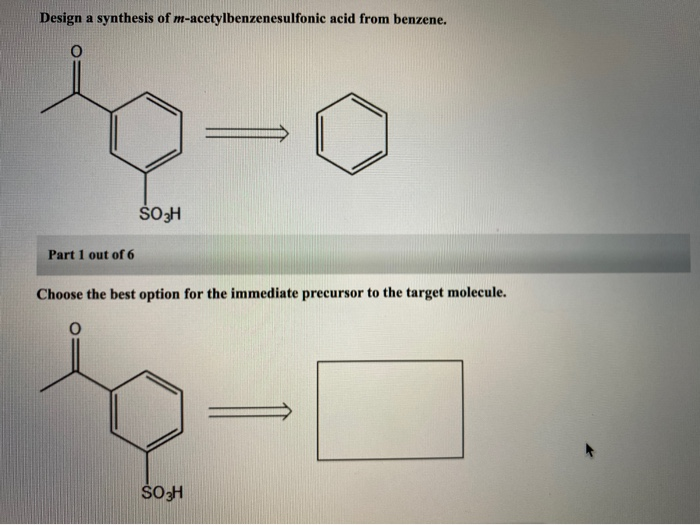
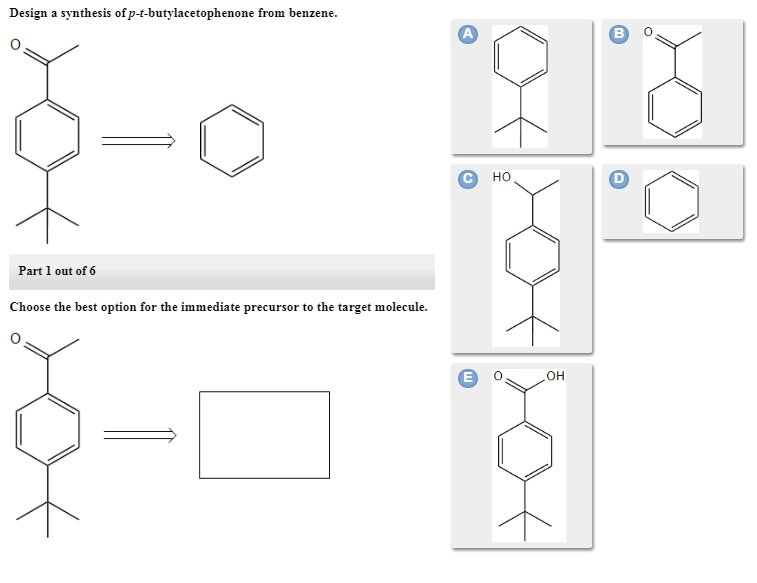
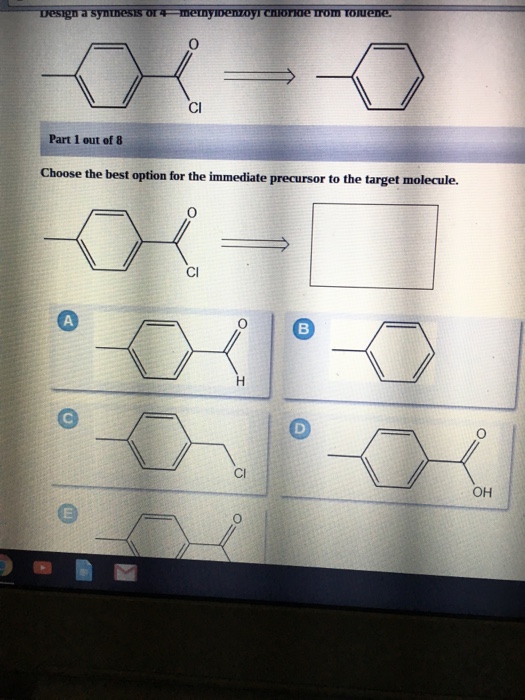
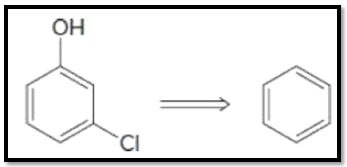
1284p1 1284p1b 1284p1a 1284p1e 1284p1d 1284p1c Dehydrohalogenation of the benzylic bromine can result in an alkene. Points Choose the best option for the immediate precursor to the target molecule. Synergistic and Competitive Groups Concept Videos All Organic Chemistry Practice Problems EAS.
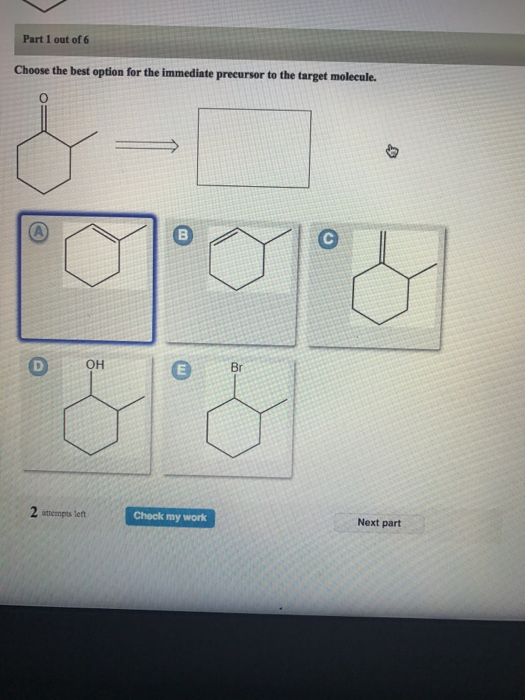
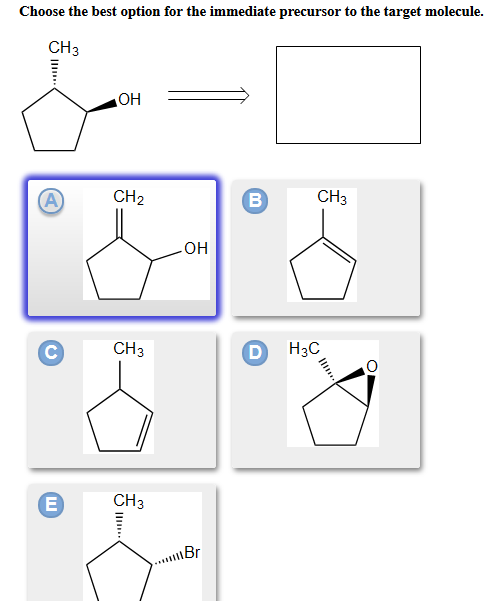
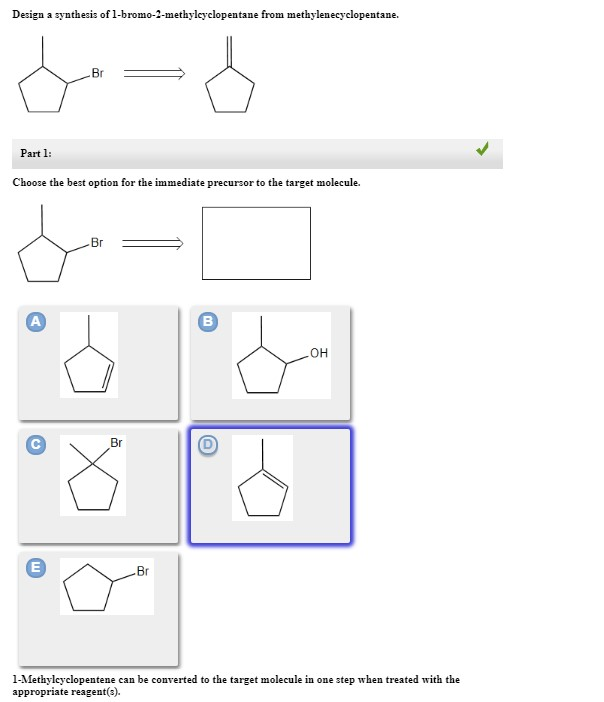
B c will give you small amounts of product the cyclopentanol will dominate as the main species since -OH is a poor leaving group under non-acidic conditions. Choose the best option for the immediate precursor to the target molecule.
Synergistic and Competitive Groups Concept Videos All Organic Chemistry Practice Problems EAS.
Hydration of the triple bond in a Markovnikov manner will result in 2-butanone. Synergistic and Competitive Groups Concept Videos All Organic Chemistry Practice Problems EAS. The retrosynthetic planning for preparation of n-butyl bromide from 2-butene is. Design a synthesis of 2-butanone from ethyne. Choose the best option for the immediate precursor to the target molecule. Choose the best option for the immediate precursor to the target molecule. Choose the best option for the immediate precursor to the target molecule. Choose the best option for the immediate precursor to the target molecule. B c will give you small amounts of product the cyclopentanol will dominate as the main species since -OH is a poor leaving group under non-acidic conditions.
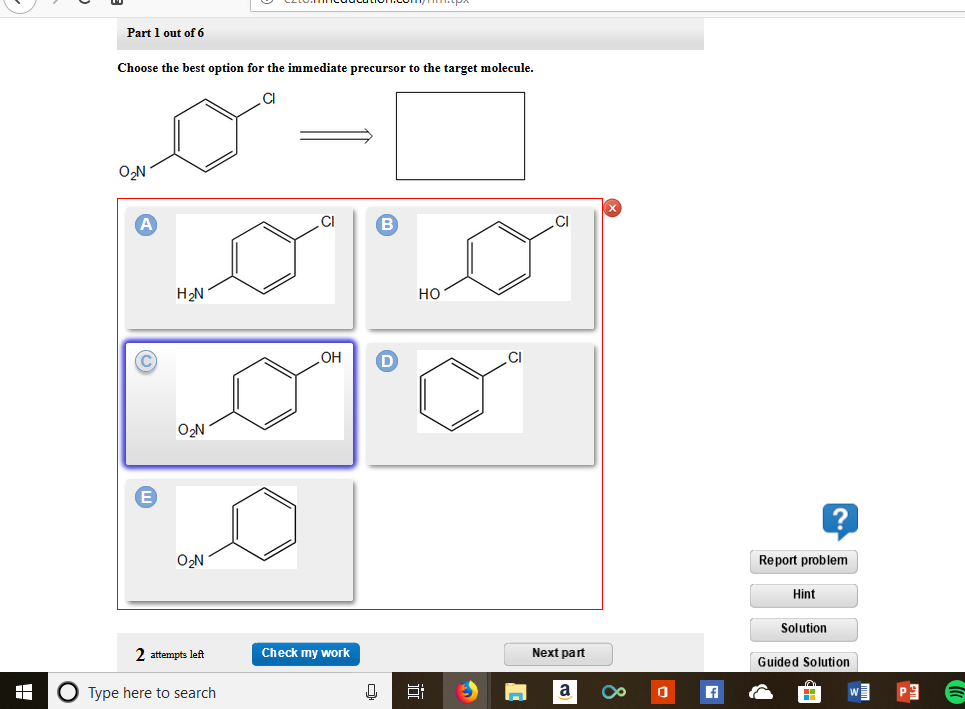
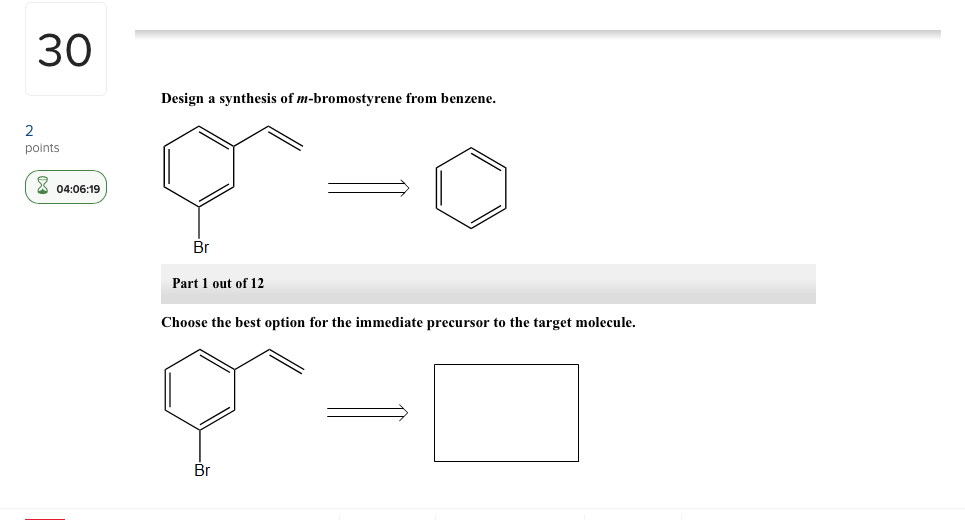
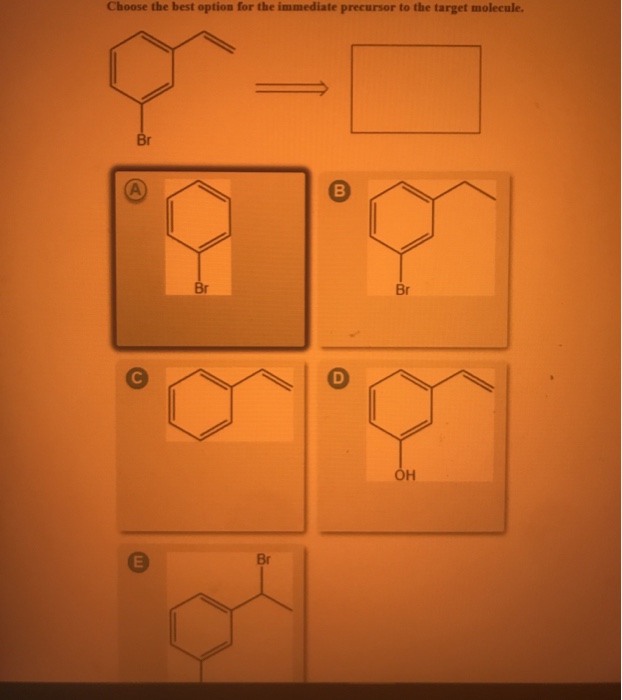
Choose the best option for the immediate precursor to the target molecule. Points Br Part 1 out of 12 Choose the best option for the immediate precursor to the target molecule. 30 Design a synthesis of m-bromostyrene from benzene. Choose the best option for the immediate precursor to the target molecule. Design a synthesis of ethyl N-ethyliminopropanoate from ethyl formate ethyl acetate and ethyl propanoate. Design a synthesis of 1-butyne from ethyne and ethanol. Choose the best option for the immediate precursor to the target molecule.























Posting Komentar untuk "Choose The Best Option For The Immediate Precursor To The Target Molecule."