Atoms That Borrow Electrons
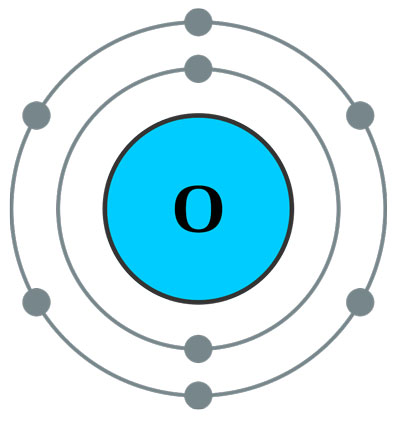
Atoms that borrow electrons. As a result oxygen acquires the electron configuration of neon. Your question asks if we could make gold by starting with super heavy atoms from reactors. Cesium trifluoride CsF3 where cesium has shared its one valence electron and two from an inner shell.
The Haber process is very important for agriculture because it converts N_2 g from the atmosphere into bound nitrogen which can be taken up and used by plants. The properties of a solid can usually be predicted from the valence and bonding preferences of its constituent atoms. A nuclear reaction in which an atoms nucleus splits to form two new atoms.
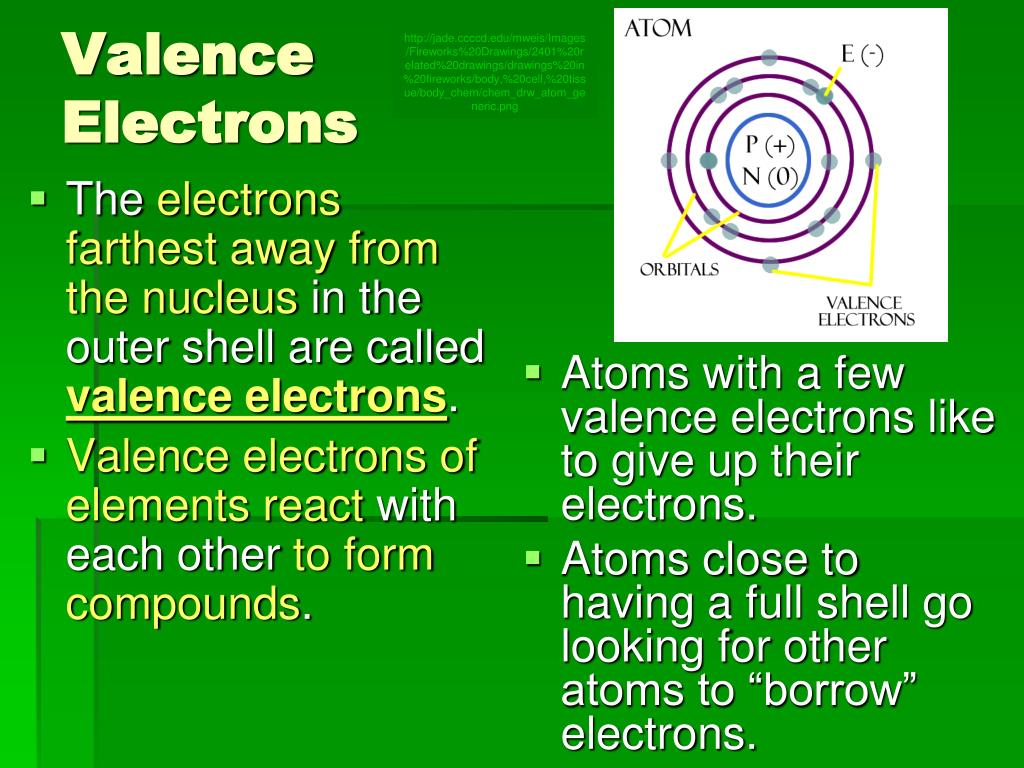
Electron shells are considered the outermost regions of the. The Haber process reaction is mathrm N_ 2 g3 mathrm H_ 2 g rightleftharpoons 2 mathrm NH_ 3 g. An element that borrow electrons in chemical reactions.
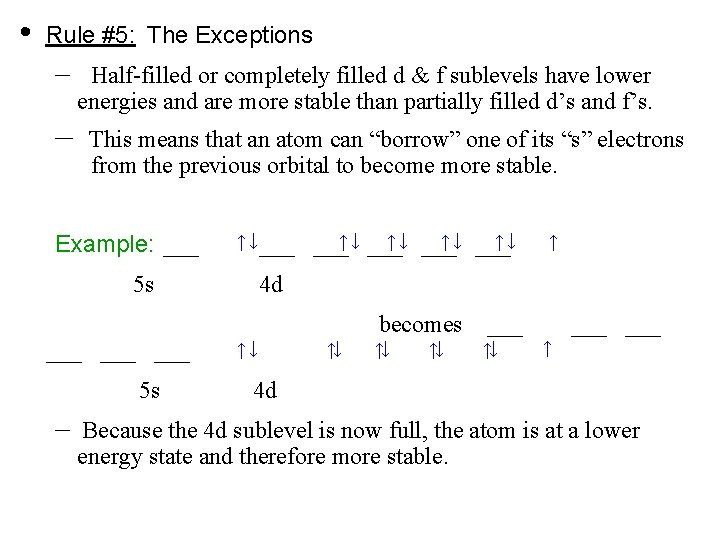
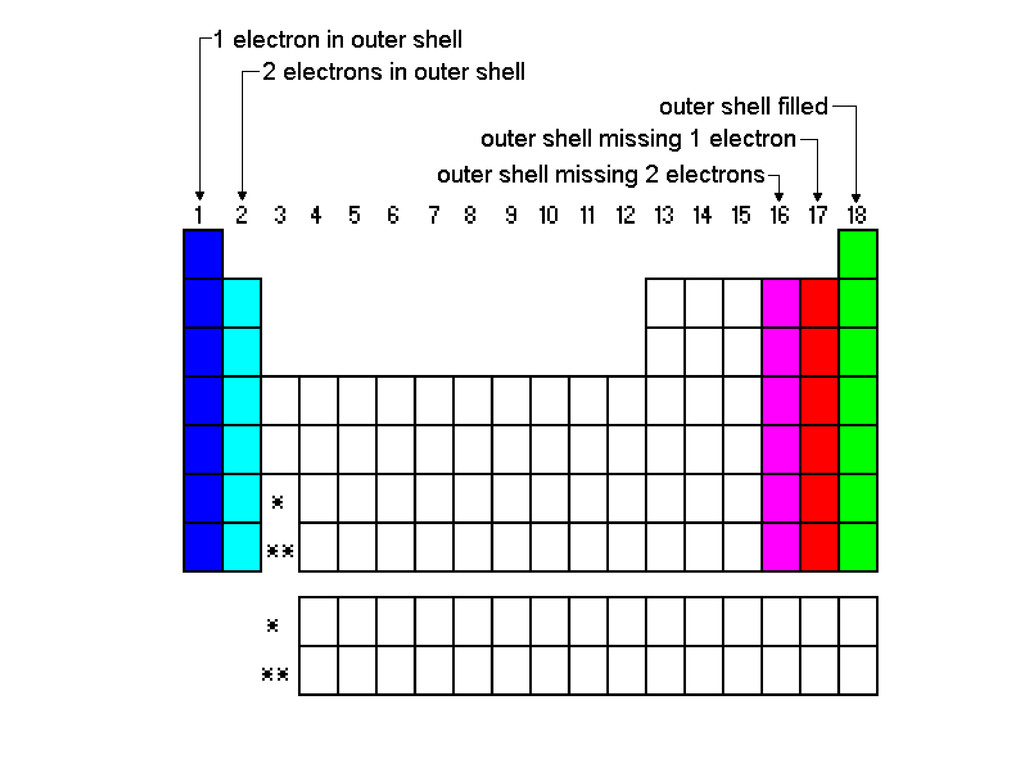
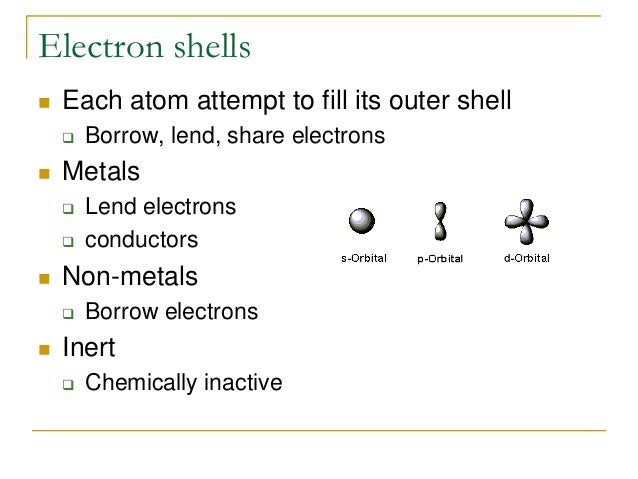
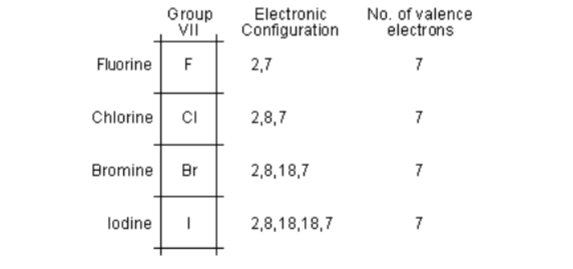
Read More on This Topic. They can try to get to eight electrons to fill up their third shell or they can give up a few electrons and have a filled second shell. Notable negative ions are chloride and fluoride.
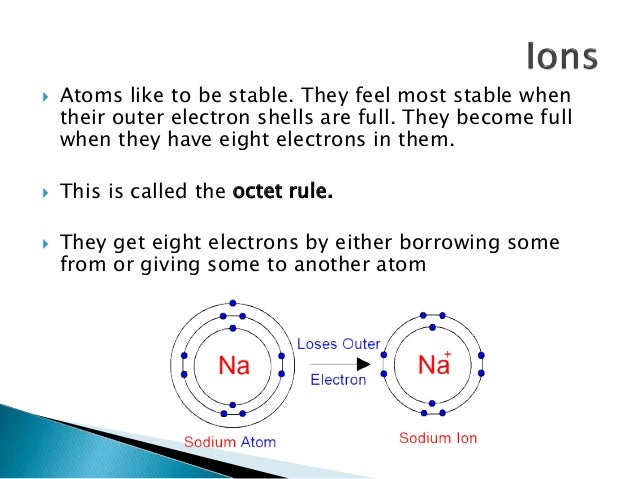
The answers already provided are or course quite right. They tend to achieve a stable octet by taking an electron from other atoms becoming negatively charged ions. But having be ATAed I thought I would add a little note.
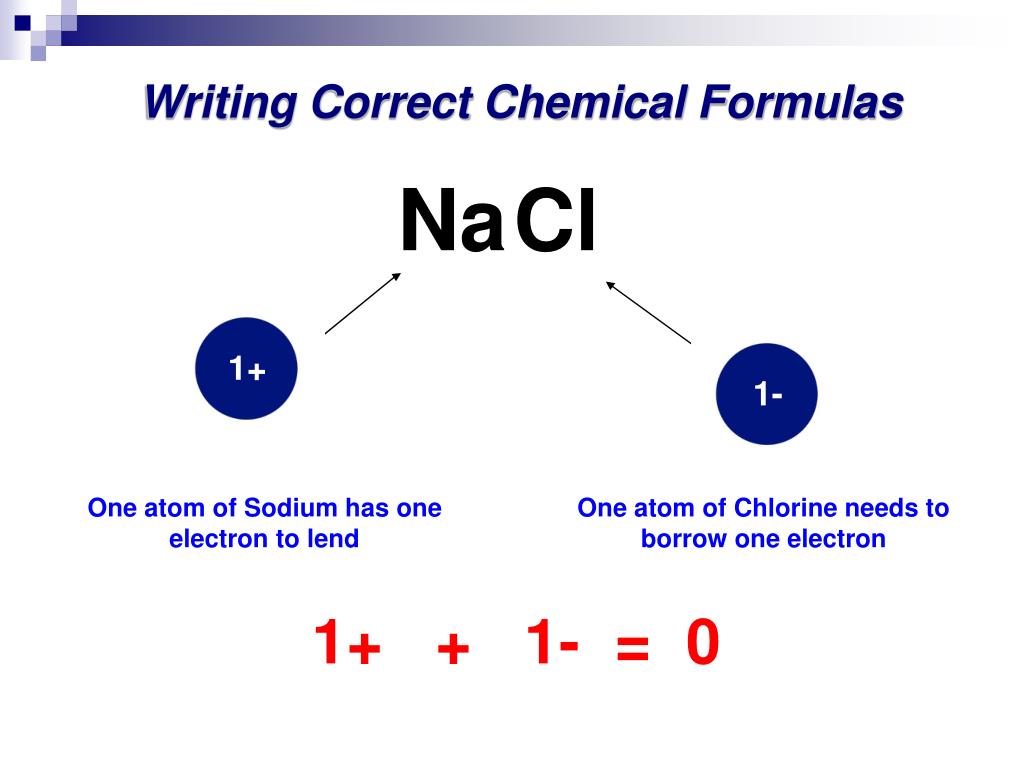
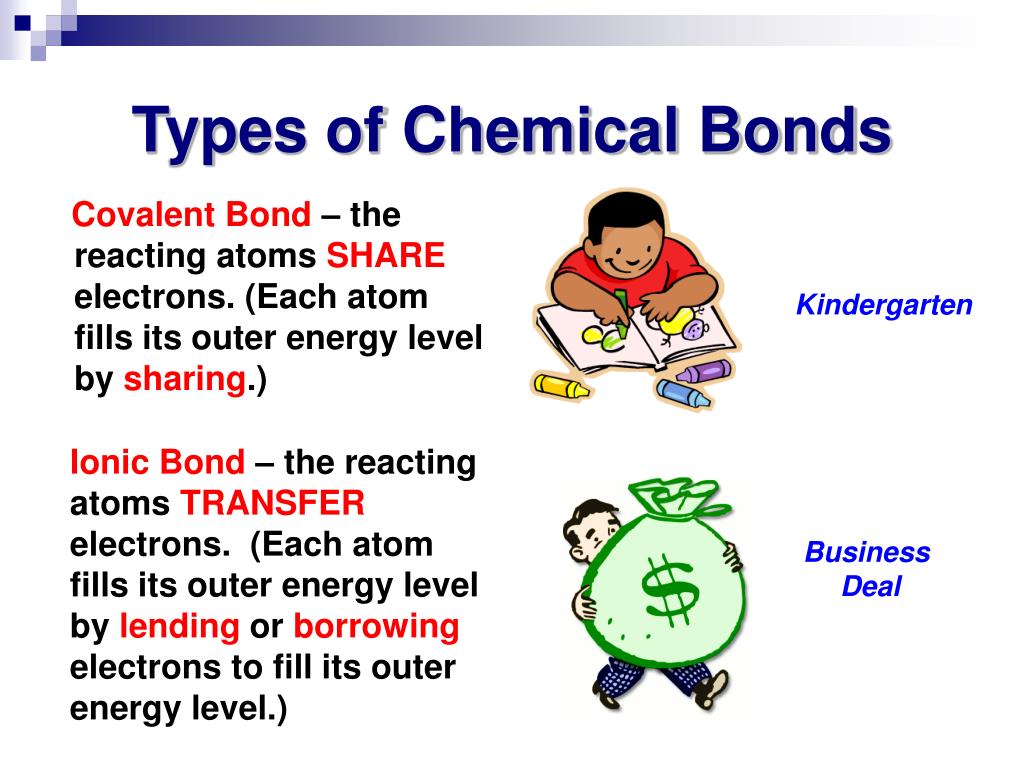
Common table salt based on this ionic bond. Carbon C as a group 14. An atom of sodium Na donates one of its electrons to an atom of chlorine Cl in a chemical reaction and the resulting positive ion Na and negative ion Cl form a stable ionic compound sodium chloride.
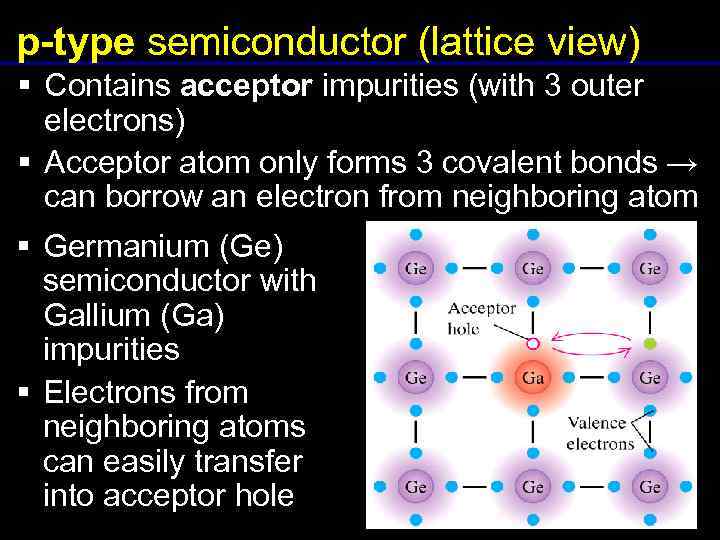
A compound has at least one metal and nonmetal. The oxygen atoms then attach to the sulfur and borrow entire electrons pairs without really contributing anything in return.
A nuclear reaction in which an atoms nucleus splits to form two new atoms.
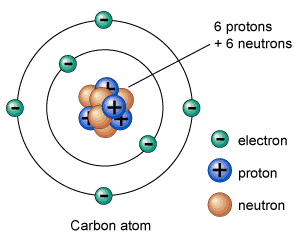
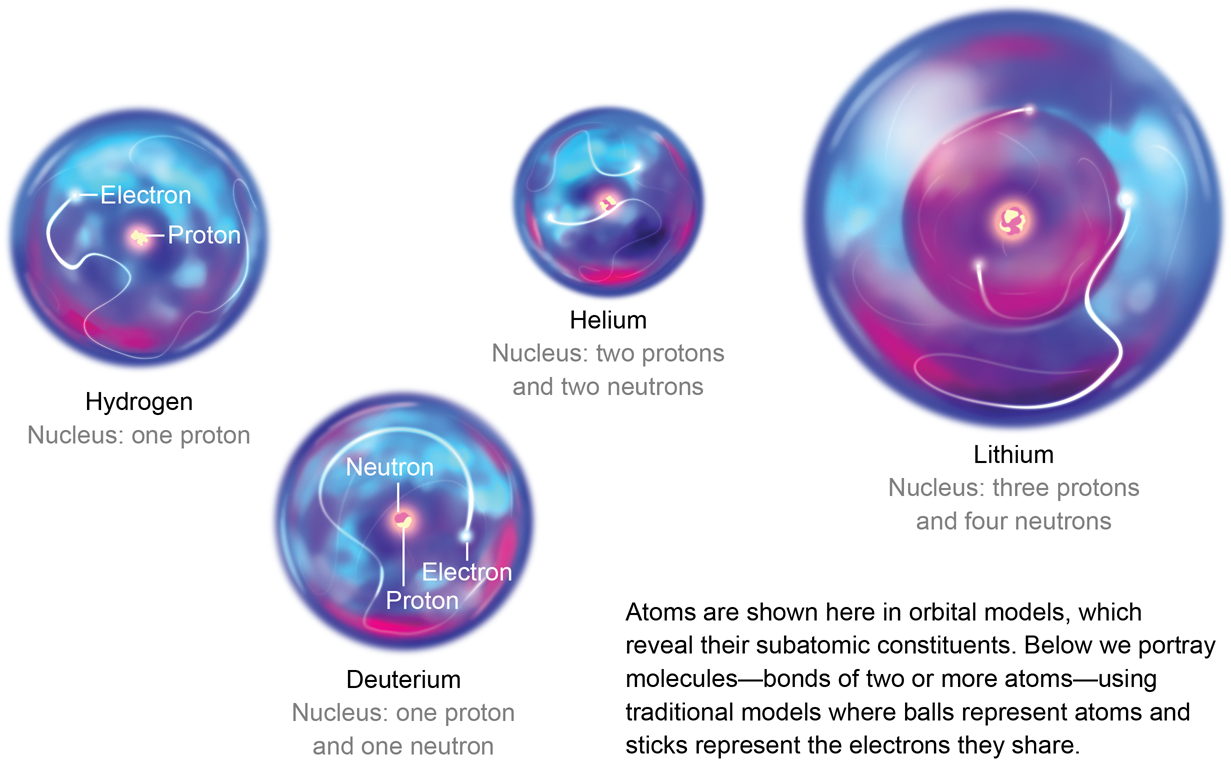
Protons positively charged and neutrons are in the nucleus centre of the atom no charge. An atom that has gained or lost one or more electrons and has a negative or positive charge Neutral Atom if atomic mass is the average one given on the periodic table and if the protons and electrons are the same then its a neutral atom. The oxygen atoms then attach to the sulfur and borrow entire electrons pairs without really contributing anything in return. Answer 1 of 6. F and Cl. Electrolytes compounds that form ions when dissolved in water. Cesium trifluoride CsF3 where cesium has shared its one valence electron and two from an inner shell. The isotope of carbon that is used for dating things in archaeology. Atoms of metals are the ones that lend the electrons.
The properties of a solid can usually be predicted from the valence and bonding preferences of its constituent atoms. Your question asks if we could make gold by starting with super heavy atoms from reactors. Atoms of non-metals are the atoms that borrow electrons. But having be ATAed I thought I would add a little note. A nuclear reaction in which the nucleus of two atoms combine to form one nucleus. An atom of sodium Na donates one of its electrons to an atom of chlorine Cl in a chemical reaction and the resulting positive ion Na and negative ion Cl form a stable ionic compound sodium chloride. Answer 1 of 6.


























Posting Komentar untuk "Atoms That Borrow Electrons"